Many international organizations and institutes participate in European projects and initiatives on research, clinical care and health policy to achieve health goals that would be unattainable when operating solely within one’s own country.
 |
| Donald Singer, Carin Uyl-de Groot, Marlies Wijsenbeek, Liese Barbier, Ken Redekop and Lytske Bakker |
The latest Fellowship of Postgraduate Medicine conference was held at Erasmus University in Rotterdam in the Netherlands on 21st June 2019 to consider European Cooperation on Healthcare. The aim was to provide a forum for discussing best practice across the above key healthcare domains.
 |
| Donald Singer, Ron de Winter, Marjan Hummel, Marcus Guardian, Lytske Bakker and Ken Redekop |
Content from the meeting will appear in the HPT journal as Editorials, commentaries, review articles and Meet the Expert reports, with associated short video interviews with speakers posted on the HPT and FPM websites.
 |
| Poster prize winner Vivian Reckers-Droog with Ken Redokop (L) and Donald Singer |
 |
| Poster presenters and European Reference Network Project Managers Olivia Spivack and Renée de Ruiter. |

Pharmacist Liese Barbier, European Medicines Agency, discussed European Medicines Agency perspectives on regulating biosimilars. She stressed the importance of batch-level information when reporting any suspected adverse drug reactions from biosimilars or corresponding biological medicines.
Jorge Gonzalez, Spain, spoke on the EU funding supported inDemand model now operating in Spain, France and Finland, with additional network partners throughout Europe. InDemand makes a virtue of needs-driven rather than technology-driven project commissioning as a more reliable approach to ensuring adoption of new approaches into clinical practice. Examples included mobile health applications to reduce weight in obese children and e-health systems to support management of women in pregnancy.
Marcus Guardian, CEO of EUnetHTA, The European Network for Health Technology Assessment discussed his organisation’s role in cross-border assessment of health technology.
Ines Hernando from the EURORDIS-Rare Diseases Europe organization discussed the initial impact of the 2017 European Reference Network Directive to improve the care of the ~ 30 million patients in Europe with rare diseases. The new European Reference Networks are already providing virtual common rare disease management support platforms for health professionals across the European region.
Marjan Hummel from Philips in Einthoven discussed early health technology assessment in the medical device industry and resulting international implications for streamlining development of new health technologies.
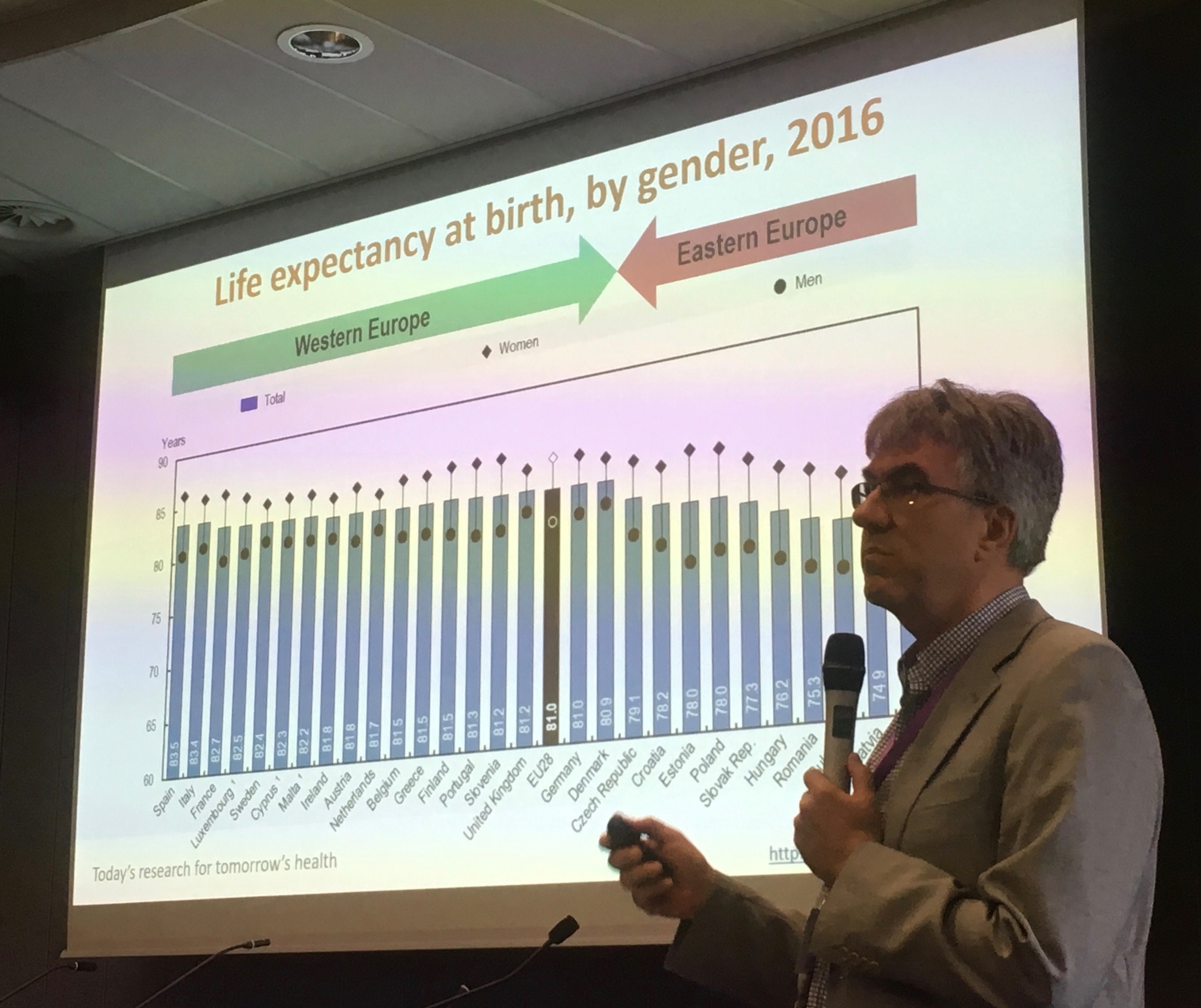
Zoltan Kalo, Professor of Health Economics at Eötvös Loránd University (ELTE) in Budapest discussed ways to improve equity in allocation of healthcare research funds by the European Union. Currently there appears to be a disproportionate allocation of EU research awards to EU15 countries. This both disadvantages research capacity development in EU13 countries and leads to a ‘brain drain’ of researchers from EU13 to EU15 research centres.

Donald Singer, President, Fellowship of Postgraduate Medicine, London discussed engaging with European health policy makers, including new networking opportunities between health professional and patient and consumer organisations and EU institutions such as the European Medicines Agency.

Carin Uyl-De Groot, head of health technology assessment at the Erasmus School of Health Policy & Management in Rotterdam discussed sustainability and affordability of innovative drugs. She described discussion with European policy makers on ways to reduce the cost of expensive biological treatments. Developing cross-border partnerships would create much greater bargaining power for purchasing medicines. For example, the EU region currently provides 40% of the market for most pharmaceuticals.
Respiratory physician Marlies Wijsenbeek from the Erasmus Medical Centre discussed patient registry development to improve management of and research into rare lung diseases, based on her work on idiopathic pulmonary fibrosis. She noted the potential value of developing cross-border patient registries for rare diseases, to ensure larger patient populations then possible within individual countries. She also illustrated some of the challenges, e.g. when common data sets are not agreed and when the same patients may feature within different registries.





